
Skin cancer
Short description
Our research group aims to improve the management of patients with skin cancer (malignant melanoma, squamous cell carcinoma, basal cell carcinoma, Merkel cell carcinoma, etc). A dozen post-doc researchers and doctoral students work in the group om clinical research projects focusing epidemiology, prevention, diagnosis and treatment of skin cancer. We also have a translational research approach with national and international collaborations within chemistry, physics, IT and mathematics.
Our research on teledermoscopy has revolutionized how patients referred for a suspicion of skin cancer are triaged today. Our focus now lies in further developing artificial intelligence and innovative imaging techniques for automated/early skin cancer diagnosis, biomarkers for Merkel cell carcinoma and improved treatment of non-melanoma skin cancer.
AI
Artificial intelligence for automated diagnosis of skin cancer
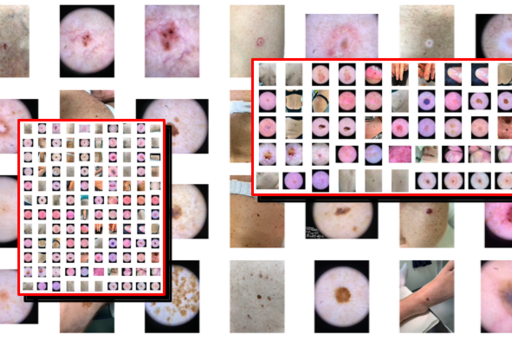
We are working on developing effective IT systems for quick and high-quality annotation and labelling of large numbers of images (macroscopic, dermoscopic and hyperspectral) of all types of benign and malignant skin tumors. The objective is to create one of the world’s largest skin image databases built for future development and training of artificial intelligence (AI) algorithms for safer and earlier diagnosis of skin cancer.
Similar to ISIC (International Skin Imaging Collaboration, www.isic-archive.com), the images in our database will be complete de-identified allowing the database to be made open access for international researchers to use and test their own AI algorithms. Other dermatology departments will also be allowed the possibility to contribute to our database in the future.
One of the first more specific areas we will be focusing on in regards to AI solutions for automated skin cancer detection is differentiating between melanoma in situ (precancerous) and invasive melanoma. Preliminary studies from our group have shown that dermatologists can correctly classify malignant melanocytic tumors as in situ or invasive in around 70% of the cases. By constructing a larger image database of approximately 1000 malignant melanocytic tumors with half being in situ and half invasive, we aim to train an AI based on deep neural networks as a diagnostic tool that will help improve this type of classification. Improving dermatologists’ diagnostic accuracy in this way will aid in deciding on the most appropriate and cost-effective excision margins for the diagnostic excision.
Hyperspectral imaging
Hyperspectral imaging for early skin cancer detection

At the department of Dermatology and Venereology, Sahlgrenska University Hospital, we are currently carrying out research on an innovative and fast imaging technique known as hyperspectral imaging, which may help us diagnose skin cancer at an early stage and recognize benign skin lesions with higher sensitivity and specificity than what is currently possible with just clinical and dermoscopic images. Hyperspectral imaging is performed with a special camera that acquires a large number of images of skin tumors using harmless light wavelengths within just a few seconds. By collecting a large number of hyperspectral images of benign and malignant skin tumors belonging to the eight most common diagnostic categories (approximately 100-200 lesions per category), an AI algorithm based on deep neural networks (machine learning) can be created, trained and tested for automated skin lesion classification.
Results from this pilot study will later be used to design an international multicenter study with our group acting as principal investigators in which approximately 1,000 images of benign and malignant skin tumors belonging to the eight most common diagnostic categories will be included. The AI algorithms will be further improved with these training sets for later testing and validation. The aim is for dermatologists, general practitioners and other physicians working with skin cancer diagnosis to improve their diagnostic accuracy when examining patients with a suspicion of skin cancer.
Short-term monitoring
Short-term monitoring of atypical melanocytic lesions
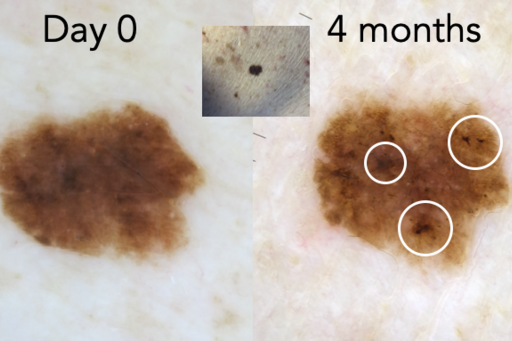
When you can’t clearly classify a pigmented lesion of melanocytic nature as benign (nevus) or malignant (melanoma), the lesion is an atypical melanocytic lesion (AML). Our research has shown that short-term monitoring of single or few AMLs in low-risk patients during a 4-month follow-up period can be used to significantly decrease the number of unnecessary excisions (<10% require surgery) while still finding melanomas at a very early or precancerous curable stage.
The method is safe and effective but requires follow-up visits since the dermoscopy equipment used has been hospital-based until now. Patients also need to travel to the hospital for new imaging and need to take time off work. By lending patients a simpler and cheaper dermoscope that can be coupled with a smartphone, patients could potentially acquire clinical and dermoscopic follow-up images in their own homes. We are therefore planning a new study analyzing the effectiveness and safety of remote dermoscopic monitoring comparing images taken by the patients themselves of selected AMLs with images taken within routine hospital care.
When performing short-term monitoring of AMLs, initial dermoscopic images are compared to the follow-up images by one or more dermatologists. We are therefore planning a new study in which a larger number of observers will make such assessments in order to describe their interobserver concordance in regards to observing structural differences in the dermoscopic images taken during short-term monitoring of AMLs.
Merkel cell carcinoma
Tumor and serum biomarkers for appearance, progression, treatment response and follow-up of Merkel cell carcinoma
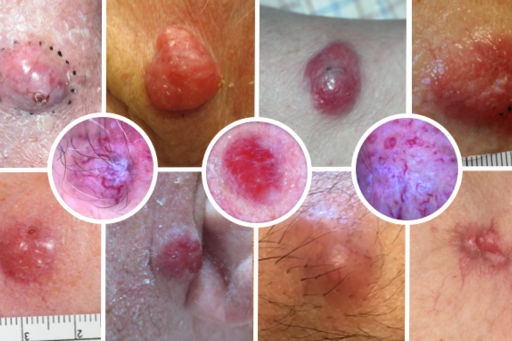
Merkel cell carcinoma is a rare but very aggressive type of skin cancer. There is a great need to better understand the mechanisms involved in the origin of Merkel cell carcinoma, what causes it to metastasize and its response to treatment. In order to improve prevention, early diagnosis, treatment and follow-up of spread disease, we are collaborating with a multidisciplinary Nordic group of dermatologists, oncologists, molecular biologists and pathologists as well as with international experts in the field such as Professor Paul Nghiem from the USA and Professor Mahtab Samimi from France. Multiple projects are ongoing with the goal of identifying new tumor biomarkers of importance in the origin and metastasis of Merkel cell carcinoma, biomarkers that can predict response to treatment and testing new serum biomarkers that can detect metastasis at a very early stage and also be used to monitor oncological treatment response.
With improved knowledge about the significance of different tumor biomarkers in regards to the origin, development and treatment response, we will be able to predict which patients will have a higher risk of metastasis providing an opportunity to treat them at an earlier stage perhaps even preventing metastasis in the first place. We may also be able to predict which patients will respond better to certain treatments allowing for individualized treatment. Furthermore, new serum biomarkers may be used instead of expensive and harmful radiological monitoring procedures.
ToF-SIMS
Time of Flight Secondary Ion Mass Spectrometry for analysis of lipids in basal cell carcinoma

Increased knowledge about the underlying genetic mutations has revolutionized how we treat various types of cancer, but during the past years changes in the composition of lipids in cancerous tissue has become a field of interest within cancer research. Changes in the composition of lipids in cancerous tissue may occur much earlier than that of proteins in cancerous cells and therefore may be the first indicators of change in skin cancer cells.
The aim of this project is to analyze the biochemical characteristics of various types of skin cancer in regards to their lipid composition by using so-called “Time-of-Flight Secondary Ion Mass Spectrometry” (ToF-SIMS). ToF-SIMS is an advanced imaging technique that can identify elements and organic molecules in various tissues.
Tissue samples from aggressive basal cell carcinoma (BCC) are being collected for ToF-SIMS analysis. A map of the lipids observed in the tissue is created and analyzed point by point. This map is then compared to histopathological images at the corresponding level. The gained knowledge and understanding about the lipid composition in skin cancer may be of great importance in improving the diagnosis of BCC and differentiating its subtypes. In addition, new treatment methods based on the lipids contained in these tumors could be developed.
Destructive treatments
Randomized controlled trials for the evaluation of destructive treatments of non-melanoma skin cancer

Basal cell carcinoma (BCC) is the most common type of skin cancer and affects over 50,000 Swedish patients per year. Squamous cell carcinoma in situ (SCCis) is a premalignant skin lesion to squamous cell carcinoma (the second most common type of skin cancer) and affects over 12,000 Swedish patients per year. One of the most common treatment alternatives for BCC and SCCis are destructive treatments such as curettage (scraping), cryotherapy (freezing with liquid nitrogen spray) and electrodesiccation (burning). Despite long traditions of using these methods, standardized treatment protocols and randomized controlled trials are lacking.
Our group is carrying out two large, prospective, randomized controlled trials comparing the effectiveness and safety between various destructive alternatives for BCC and SCCis.
In the BCC study, three treatment groups have been established: A) superficial BCCs located between the neck and the knees, B) other non-morpheaform BCCs between the neck and the knees, and C) non-morpheaform BCCs below the knees. In group A, we compare curettage alone vs. cryotherapy alone; in group B, curettage & cryotherapy with 1 vs. 2 cycles; and in group C, curettage alone vs. curettage and electrodesiccation with 2 cycles.
The SCCis study includes two treatment groups: A) SCCis located above the knee and B) SCCis below the knee. In group A, we compare curettage alone vs. cryotherapy alone and in group B, we compare curettage alone vs. curettage and electrodesiccation with 2 cycles.
In both studies, wound healing is assessed after 4 weeks, clinical response after 3-6 months and recurrence rates after 1, 3 and 5 years, respectively.
Photodynamic therapy
Photodynamic therapy with simulated daylight for treatment of superficial non-melanoma skin cancer

Photodynamic therapy (PDT) is an established treatment method for superficial non-melanoma skin cancer (actinic keratosis, superficial basal cell carcinoma and squamous cell carcinoma in situ). Treatment is carroed out in two steps. First, a cream containing a photosensitizer (5-aminlevulinic acid, ALA, or methyl aminolevulinate, MAL) is applied on the lesion and then the area is illuminated with either daylight after 30 minutes for 2 hours (daylight PDT) or with red LED lights after 3 hours for 8-10 minutes (conventional PDT). The biggest drawback with conventional PDT is the pain that most patients feel during the illumination with the red light. Daylight PDT isn’t as strong causing less pain. However, daylight PDT is weather-dependent and it is difficult to control and standardize the total light dose provided. In Sweden and many other countries at high northern latitudes, daylight PDT is also difficult to carry out due to low temperatures outdoors, rain and insufficient light during large periods of the year. It can also seem a bit paradoxical to treat sun-damaged skin with even more ultraviolet radiation. At the Department of Dermatology at Sahlgrenska University Hospital we have installed a special lighting system in a small room in order to provide simulated daylight PDT (SD-PDT) with standardized doses of harmless light indoors.
Our group is carrying out a large, prospective, randomized controlled trial comparing the effectiveness and safety between SD-PDT and conventional PDT for the treatment of superficial non-melanoma skin cancer (actinic keratosis, superficial basal cell carcinoma and squamous cell carcinoma in situ) as well as the adverse events caused by the treatments. The goal is to provide similar effectiveness with SD-PDT compared to conventional PDT against superficial non-melanoma skin cancers without as much pain.
The study has three treatment groups including patients with: A) multiple actinic keratosis with symmetric distribution, B) superficial basal cell carcinoma and C) squamous cell carcinoma in situ. Patients in group A are randomized to SD-PDT on one half of the treatment area and conventional PDT on the other (split face design). Each lesion for patients in groups B and C are randomized to receive either SD-PDT or conventional PDT. Follow-up visits are planned after 3-6 months to assess treatment success and after 1 and 2 years to determine the risk of recurrence.
Incomplete excisions
Risk factors for incomplete excisions of skin cancer

Dermatologists, primary care physicians, ENT doctors, plastic surgeons and other surgical specialists excise benign and malignant skin tumors. Depending on the suspected diagnosis (e.g. dysplastic nevus, basl cell carcinoma, squamous cell carcinoma or malignant melanoma), tumors are excised with different margins to ensure complete removal and to make sure that national guidelines are followed.
Through a retrospective analysis of histopathological reports and clinical patient histories from 2014-2020, we are studying the risk factors associated with incomplete excisions of, amongst others, melanocytic lesions, squamous cell carcinoma, squamous cell carcinoma in situ and basal cell carcinoma. Examples of such clinical and/or pathological risk factors for incomplete excisions of skin tumors can be the histopathological subtype, the tumor location or size, the physician’s specialty or experience, if recommended surgical margins were used or not, etc. By analyzing the shortcomings in the clinical practice of surgically removing different skin tumors, new national and international guidelines and recommendations can be developed that can guarantee a higher number of curative treatments on the first attempt. This will potentially result in less suffering for patients, fewer unnecessary re-excisions, lower compensation costs for healthcare journeys and sick leave as well as fewer wound care visits with nurses. A lower incomplete excision rate will also result in fewer histopathological analyses minimizing costs and workloads for pathologists who are already in short supply in Sweden.
Melanocytic density
Immunohistochemical detection of melanocytes in lentigo maligna

Lentigo maligna is a precancerous lesion which usually appears on chronically sun-damaged skin. If left untreated or treated inappropriately, it can lead to invasive melanoma. The difficulty of treating these tumors is the fact that the tumor margins are often very difficult to demarcate clinically, dermoscopically and even histopathologically. Despite treating lentigo maligna with potentially mutilating surgical excision with a 5-10 mm margin, it is not uncommon that they are incompletely excised. Furthermore, pathologists also have problems deciding whether or not clear margins have been obtained or not microscopically. There is also a lack of reproducibility in criteria for histopathological identification and assessment of the number of melanocytes close to the surgical margin. New immunohistochemical markers like SOX10 can help pathologists detect melanocytes with higher accuracy.
In this project, we will analyze the differences in melanocytic density counts in the proximity of the surgical borders of excised lentigo maligna cases comparing digital slides with traditional hematoxylin-eosin staining with immunohistochemical staining with SOX10. The interobserver concordance between five different observers will be determined. Future projects will focus on whether or not the melanocytic density counts at the excision borders can help predict the risk of recurrence following surgery in cases with uncertain margin control comparing traditional hematoxylin-eosin staining and other immunohistochemical stains including SOX10. This could potentially lead to fewer unnecessary and potential mutilating re-excisions and improve the follow-up recommendations for patients treated for lentigo maligna.
Mohs surgery
Improved indications for Mohs micrographic surgery for facial basal cell carcinoma
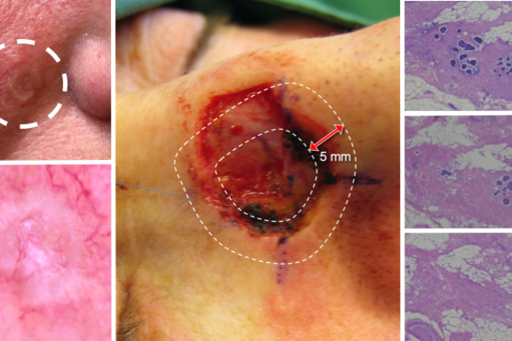
The Mohs micrographic surgery (MMS) center at the Department of Dermatology, Sahlgrenska University Hospital is the largest and oldest of the three MMC centers that exist in Sweden, where MMS is solely used for the treatment of aggressive and/or recurrent cases of facial basal cell carcinoma (BCC). MMS involves repeated surgical removal of the skin tumor with intraoperative margin control based on stained horizontal sections on frozen fresh tissue ensuring clear surgical margins prior to reconstruction. The technique guarantees complete removal of otherwise difficult-to-treat aggressive or recurrent tumors while also sparing as much healthy tissue as possible in critical areas such as the nose, eyelids, ears and lips.
Through retrospective analysis of data from our local MMS register including all patients with BCC treated with MMS in Gothenburg since 1983, we will study ways of improving the indications for MMS and its cost-effectiveness in order for the right patients to be treated with the right alternative directly. We will compare the number of MMS stages required to achieve complete tumor removal, the final defect sizes and complexity of the reconstructive methods needed depending on the tumor characteristics prior to surgery (e.g. comparing primary BCCs with previously treated BCCs that have been incompletely excised or have recurred). We are also carrying out another complementary study looking at the dermoscopic criteria for aggressive BCC in order for physicians treating BCCs to be able to recognize difficult-to-treat BCCs and demarcate the tumor with higher precision prior to choosing the optimal treatment alternative.
Rare skin cancer
Epidemiology of rare skin cancers in Sweden

Beyond the three most common types of skin cancer (basal cell carcinoma, squamous cell carcinoma and melanoma) there are a group of rare but important types of skin cancer including Merkel cell carcinoma, Kaposi sarcoma, atypical fibroxantoma, dermatofibrosarcoma protuberans, extramammary Paget’s disease, etc. This is a heterogeneous group of tumors often included in the category of “non-melanoma” skin cancers together with basal cell carcinomas and squamous cell carcinomas. Despite also arising in the skin, each of these cancer types behaves differently biologically and require different treatment approaches. Today, little is known about the trends regarding changes in their incidence, which patients are at risk, what body parts are affected most and the mortality they cause.
In this project, we are analyzing data from the Swedish Cancer Register regarding these rare skin cancer types from 1993 until today, retrospectively analyzing their incidence and abovementioned clinical parameters. We have already shown that the incidence of Merkel cell carcinoma has doubled in Sweden during the past two decades. We are now working on similar data regarding Kaposi sarcoma, in which the incidence may have been affected negatively thanks to the improved treatments for HIV-infected patients whom seldom develop AIDS nowadays.
More members of the group
Johan Dahlén Gyllencreutz
Noora Neittaanmäki
Helena Svensson
Sofia Berglund
Hanna Ceder
Kristina Maltese
Maja Modin
Karolina Wojewoda
Martin Gillstedt