TAT - Clinical Studies
Short description
All research performed by the Targeted Alpha Therapy Group, irrespective of weather it concerns basic radiobiological, physical or chemical issues, has the final goal of becoming beneficial for the patient. The last 10 years of research by the group has resulted in a phase I study being completed (Journal of Nuclear Medicine 2009;50:1153–1160).
Below follows a short presentation of the methods, results, and conclusion from that study.
The phase I study
The alpha-particle emitter 211At labeled to a monoclonal antibody (mAb) has proven safe and effective in treating microscopic ovarian cancer in the abdominal cavity of mice. Beginning in 2005, women in complete clinical remission following second-line chemotherapy for recurrent ovarian carcinoma were enrolled in the study. The aim was to determine the relevant pharmacokinetics for assessing absorbed dose to normal tissues and investigating the toxicity.
Methods
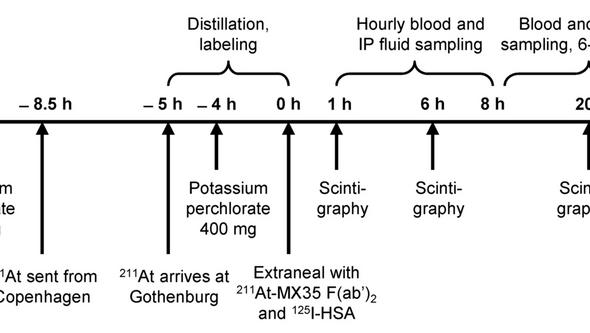
Nine patients underwent laparoscopy 2–5 d before the therapy; a peritoneal catheter was inserted and the abdominal cavity was inspected to exclude the presence of macroscopic tumor growth or major adhesions. Peritoneal scintigraphy was done using 99mTc-LyoMAA to study the fluid distribution in the abdominal cavity. Approximately 500 MBq of 211At was labeled to 0.7 mg of MX35 F(ab’)2 using the reagent N-succinimidyl 3(trimethylstannyl) benzoate. Fifty MBq (3 patients) or 100–200 MBq (6 patients) in 1–2 L of Extraneal was infused via the peritoneal catheter. Gamma camera whole-body scans were acquired at 1, 6, 12, and 20 h (occasionally up to 48 h) after infusion and a single-photon emission computed tomography (SPECT) scan was acquired at 3–6 h. Samples of blood, urine, and peritoneal fluid were collected at 1–48 h. Haematology as well as renal and thyroid function were followed for a median of 23 months.
Results
Pharmacokinetics and dosimetric results were related to the initial activity concentration (IC) of the infused solution. The decay-corrected activity concentration decreased with time in the peritoneal fluid to 50% IC at 24 h, increased in serum to 6% IC after 30 h, and increased in the thyroid to 127 ± 63% IC at 20 h without blocking and less than 20% IC with blocking. No other organ uptakes could be detected. The cumulative urinary excretion was 40 kBq/(MBq/L) at 24h. The estimated absorbed dose to the peritoneum was 15.6 ± 1.0 mGy/(MBq/L), to red bone marrow was 0.14 ± 0.04 mGy/(MBq/L), to the urinary bladder wall was 0.77 ± 0.19 mGy/(MBq/L), to the unblocked thyroid was 24.7 ± 11.1 mGy/(MBq/L), and to the blocked thyroid was 1.4 ± 1.6 mGy/(MBq/L) (mean ± 1 SD). No adverse effects were observed either subjectively or in laboratory parameters.
Conclusion: This study indicates that by intraperitoneal administration of 211At-MX35 F(ab’)2 it is possible to achieve therapeutic absorbed doses in microscopic tumor clusters without significant toxicity.