Fluorescence Resonance Energy Transfer
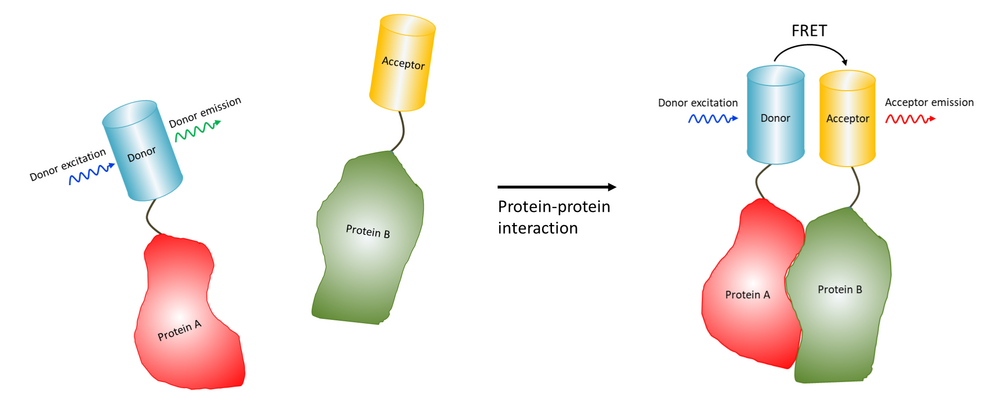
Fluorescence Resonance Energy Transfer (FRET) enables the study of molecular interactions by exciting a donor fluorophore, which transfers the energy to an acceptor fluorophore if they are in close proximity.
Fluorescence resonance/Förster energy transfer (FRET) is the radiationless transfer of energy between two molecules, which can occur if they are very close to each other (< 10 nm).
Therefore FRET makes it possible to measure if two molecules, for example a ligand and a receptor, interact with eachother. For FRET to happen the fluorescence emission spectrum of the donor has to overlap with the adsorption spectrum of the acceptor and the donor, and the transition dipole orientations must be approximately parallell.
The transfer can be measured by looking at the quenching of fluorescence of the donor in presence and absence of the acceptor, the so-called acceptor bleaching method, or directly by excitation of the donor and then looking at the fluorescence of the acceptor, so-called sensitised emission.
At CCI the acceptor bleaching method can be performed at any confocal microscope. The sensitized emission method can also be performed using a widefield fluorescence microscope.

FRET experiement in C. elegans by our user Ranjan Devkota.
(A-B) Confocal images showing the expression of a tagged protein in gonad sheath cells. Images were acquired in (A) the GFP channel (excitation GFP/emission GFP) and (B) the mCherry (excitation mCherry /emission mCherry) channel. (C) FRET image acquired in the FRET channel (excitation GFP /emission mCherry). (D) Normalized FRET image generated by the PixFRET plug-in using ImageJ. The scale represents the distribution of pixel intensities in the NFRET image.